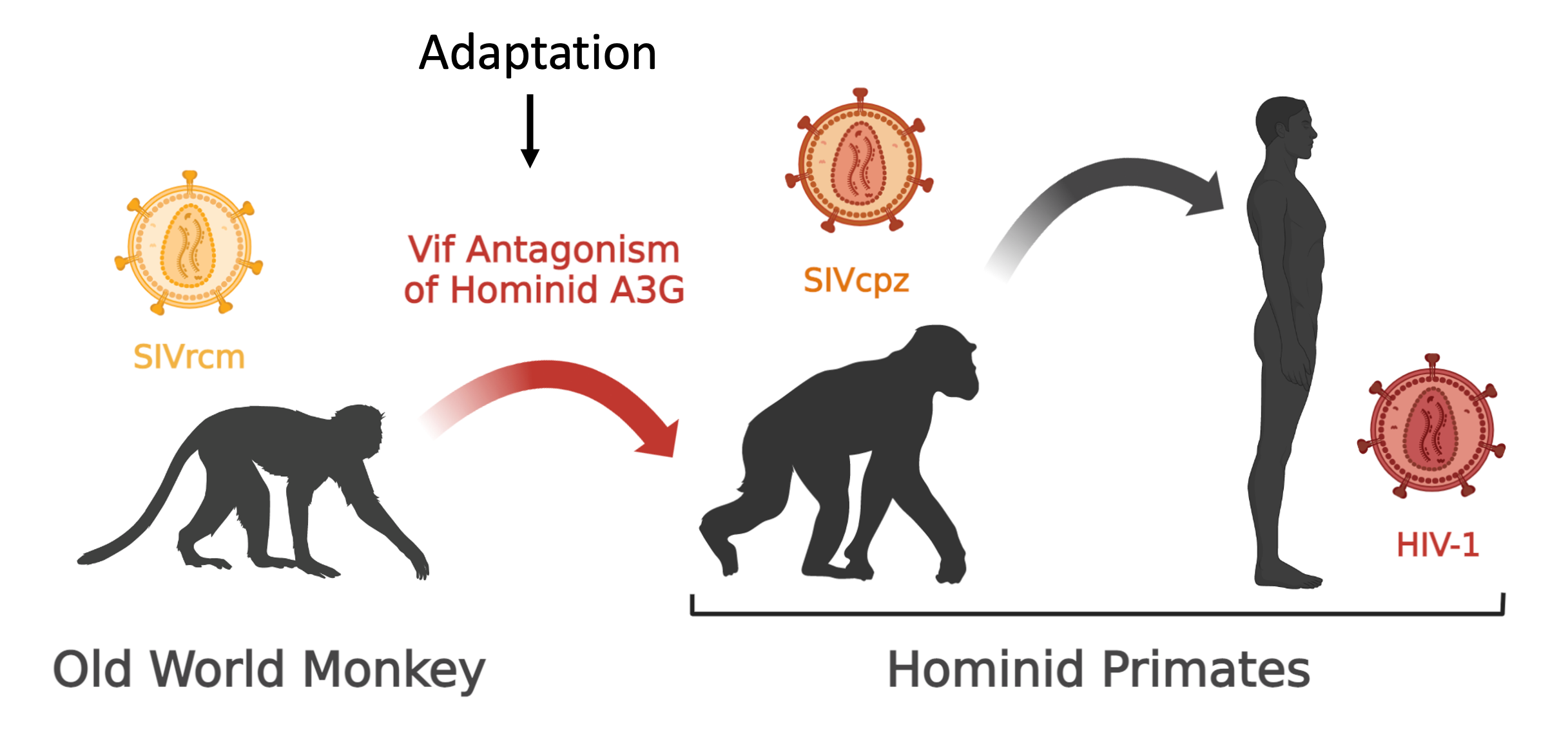
We are interested in how viruses suppress innate immunity and how hosts escape suppression during evolution. By combining biochemistry, structural biophysics and viral genetics we seek to bridge the gap between how subtle changes in primary sequence affect protein interactions that determine whether host or virus survives. We study the host-pathogen conflict between the lentiviral protein Vif and host antiviral APOBEC3 family members. This work is significant because it could inform upon antiviral therapies and explain how viruses cross species giving rise to pandemics such as AIDS. Learn more
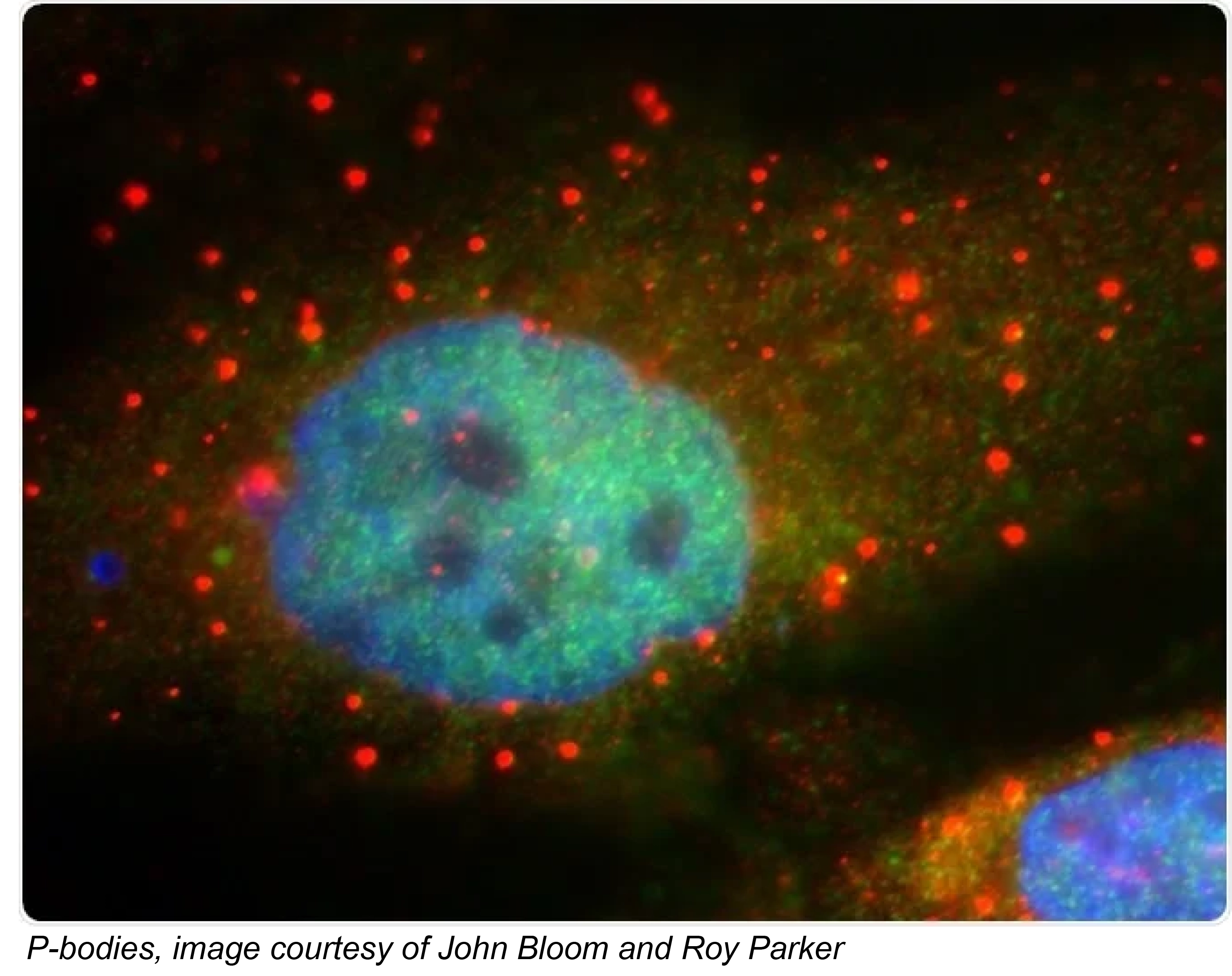
The regulated destruction of mRNA transcripts plays an important role in controlling cellular gene expression. Like protein degradation, RNA decay pathways are hijacked during viral infection. A critcal step in the 5’-3’ mRNA decay pathway is removal of the protective 5’ cap by the dynamic, multiprotein decapping complex, which commits the transcript to rapid degradation. Our lab is developing a structural-level understanding of how the decapping complex catalyzes mRNA cap cleavage ; how decapping activity is regulated by protein-protein interactions; and how 5’-3’ decay pathways are hijacked by viruses. We are also investigating how liquid-liquid phase separation provides an additional layer of regulation. Learn more